Gases y cambio de fase
Índice
Gases
- Considere un tanque de volumen $V=50{,}0\ \mt{l}$ que contiene $16{,}9\ \mt{kg}$ de argón cuando la temperatura es de $15{,}0^\circ\mt C$.
- Determine la cantidad de sustancia de argón contenida en el tanque y su volumen molar.
- Calcule la presión del argón como si fuese un gas ideal.
- Van der Waals
$$\left(p+\frac{a}{{\bar v}^2}\right)(\bar v-b)=RT$$
$a_{_{\ce{Ar}}}=1{,}630\times10^{-2}\ \mt{\frac{Pa\,m^6}{mol^2}}$
$b_{_{\ce{Ar}}}=3{,}201\times10^{-5}\ \mt{\frac{m^3}{mol}}$ - Redlich - Kwong
$$\left(p+\frac{a}{\sqrt{T}\,\bar v(\bar v+b)}\right)(\bar v-b)=RT$$
$a_{_{\ce{Ar}}}=0{,}2029\ \mt{\frac{Pa\,m^6\sqrt{K}}{mol^2}}$
$b_{_{\ce{Ar}}}=2{,}219\times10^{-5}\ \mt{\frac{m^3}{mol}}$
Gases ideales
- Un tanque contiene un volumen de $0{,}100\ \mt{m^3}$ de gas helio ($\ce{He}$) a $150\ \mt{atm}$
- Si el tanque se vacía por completo, calcule cuántos globos puede inflar el tanque si cada globo lleno es una esfera de $0{,}300\ \mt m$ de diámetro a una presión de $1{,}20\ \mt{atm}$.
- Si el volumen específico de los globos es de $v=1{,}50\ \mt{m^3/kg}$, obtenga la masa total de helio.
- Teniendo en cuenta la pregunta anterior, calcule el volumen molar $\bar v$ de helio en el tanque.
- En un tanque de $4{,}7\ \mt l$ se tienen $0{,}90\ \mt{kg}$ de oxígeno molecular ($\ce{O2}$) a $0{,}0^\circ\mt C$. Durante un incendio la temperatura del tanque se eleva a $500^\circ\mt C$. Determine
- La cantidad de sustancia de oxígeno molecular.
- El volumen molar del oxígeno molecular.
- La presión antes del incendio.
- La presión durante el incendio.
- Una sala de $24{,}0\ \mt{ft}$ de largo, $18{,}0\ \mt{ft}$ de ancho y $8{,}00\ \mt{ft}$ de alto contiene aire cuya masa molar promedio es de $28{,}97\ \mt{lb_m/lbmol}$. Si la temperatura del cuarto se eleva de $65{,}0^\circ\mt F$ a $80{,}0^\circ\mt F$,
- ¿Qué proceso es este?
- Obtenga el cambio de volumen que experimenta el aire inicialmente contenido en la sala a $65{,}0^\circ\mt F$.
- Calcule la masa del aire que escapará de la sala.
- A $25{,}0\ \mt{m}$ bajo la superficie del mar ($\rho_\text{mar}=1\,025\ \mt{kg/m^3}$), donde la temperatura es de $5{,}00^\circ\mt C$, un buzo exhala una pequeña burbuja de aire que tiene un volumen de $1{,}00\ \mt{cm^3}$. Si la temperatura en la superficie del mar es igual a $20{,}0^\circ\mt C$ ¿Cuál es el volumen de la burbuja justo antes de que se rompa en la superficie?
- En el diagrama de Clapeyron ($p\bar v$) se representan cinco procesos $ab$, $bc$, $ad$, $dc$ y $ac$, correspondientes a un gas ideal. Nótese que la línea morada es una isoterma.

- Esboce los mismos procesos en un diagrama $pT$ y en un diagrama $T\bar v$
- Obtenga todas las coordenadas termodinámicas de los estados $a$, $b$, $c$ y $d$.
- Calcule el volumen en el estado $a$, si el sistema consiste en $4{,}00\ \mt{kmol}$ de hidrógeno molecular $\ce{H_2}$.
- Encuentre la masa del hidrógeno molecular $\ce{H_2}$.
Titulación
- Determine la fase o fases constituido por $\ce{H_2O}$, localice los estados sobre diagramas $p$ v/s $v$ y $T$ v/s $v$ en las siguientes condiciones
- $p = 500\ \mt{kPa}$, $T = 190{,}0^\circ\mt C$.
- $p = 5{,}00\ \mt{MPa}$, $T = 263{,}9^\circ\mt C$.
- $p = 9{,}30\ \mt{bar}$, $T = 180{,}0^\circ\mt C$.
- $p = 18{,}3\ \mt{MPa}$, $T = 120{,}0^\circ\mt C$.
- $p = 1{,}50\ \mt{kPa}$, $T = -17{,}3^\circ\mt C$.
- $p = 75{,}0\ \mt{psi}$, $T = 307{,}6^\circ\mt F$.
- $p = 1{,}08\times 10^4\ \mt{psf}$, $T = 392{,}4^\circ\mt F$.
- $p = 350\ \mt{psi}$, $T = 405{,}0^\circ\mt F$.
- $p = 98{,}4\ \mt{psi}$, $T = 340{,}0^\circ\mt F$.
- $p = 15{,}0\ \mt{psi}$, $T = -10{,}5^\circ\mt F$.
Indicación: Busque los datos del cambio de fase del agua en las tablas de la sección Propiedades del agua saturada.
- Determine el título de las mezclas bifásicas líquido-vapor de:
- $\ce{H_2O}$ a $200^\circ\mt C$ con un volumen específico de $9{,}50\times 10^{-2}\ \mt{m^3/kg}$.
- Refrigerante 134a a $30{,}0\ \mt{psi}$, con un volumen específico de $1{,}20\ \mt{ft^3/lb_m}$.
Indicación 1: Revise los datos de la sección Constantes, datos y factores de conversión.
Indicación 2: El refrigerante 134a es una sutancia química ampliamente usado en la industria. Se trata de $1{,}1{,}1{,}2-$Tetrafluoroetano ($\ce{CH_2FCF_3}$).
- El volumen específico de líquido y vapor saturados del nitrógeno a $100\ \mt K$ son, $v_f = 1{,}452\times 10^{-3}\ \mt{m^3/kg}$ y $v_g = 31{,}31\times 10^{-3}\ \mt{m^3/kg}$.
Determine el título de $22\ \mt{kg}$ de nitrógeno de una mezcla bifásica líquido-vapor a $100\ \mt K$ en un depósito cuyo volumen es $0{,}50\ \mt{m^3}$. - En un depósito cuyo volumen es $0{,}21\ \mt{m^3}$ se almacena amoníaco. Si se halla como líquido saturado a $20^\circ\mt C$, determine
- La masa de amoníaco.
- La presión del amoníaco.
Indicación: El amoníaco es un gas incoloro de olor penetrante y desagradable ($\ce{NH_3}$).
- Una mezcla bifásica líquido vapor de $\ce{H_2O}$ tiene una temperatura de $500^\circ\mt F$ y un título de $75\%$. Si la mezcla ocupa un volumen de $1{,}80\ \mt{ft^3}$. Determine las masas de líquido y vapor saturados.
- Una mezcla bifásica líquido vapor de cierta sustancia se encuentra a una presión de $150\ \mt{bar}$ y ocupa un volumen de $0{,}20\ \mt{m^3}$. Si las masas de líquido saturado y vapor saturado presente en la mezcla son $3{,}8\ \mt{kg}$ y $4{,}2\ \mt{kg}$, respectivamente, determine el volumen específico de la mezcla.
Constantes, datos y factores de conversión
- Aceleración de gravedad estándar
$g=9{,}81\,\mt{m/s^2}= 32{,}2\,\mt{ft/s^2}$. - Presión atmosférica estándar
$p_\text{atm}\equiv 1\,\mt{atm}\equiv 101\,325\,\mt{Pa}=2\,116{,}2\,\mt{lb/ft^2}=14{,}696\ \mt{psi}.$ - Temperatura del cero absoluto
$T_{0\,\mt K}\equiv 0\ \mt K\equiv 0\ \mt R\equiv -273{,}15^\circ\mt{C}\equiv -459{,}67^\circ\mt{F}.$ - Constante Universal de los gases
$R=8{,}314\ \mt{\frac{Pa\cdot m^3}{mol\cdot K}}=1\,545\ \mt{\frac{ft\cdot lb_f}{lbmol\cdot R}}$ - Masa atómica del argón $\ce{Ar}$
$M_{\ce{Ar}}=39{,}948\ \mt{g/mol}$. - Masa atómica del helio $\ce{He}$
$M_{\ce{He}}=4{,}00\ \mt{g/mol}$. - Masa atómica del oxígeno $\ce{O}$
$M_{_{\ce{O}}}=16{,}00\ \mt{g}$. - Masa molar promedio del aire
$M_\text{aire}=28{,}97\ \mt{lb_m/lbmol}$. - Masa atómica del hidrógeno $\ce{H}$
$M_{\ce{H}}=1{,}008\ \mt{g/mol}$. - Propiedades líquido-vapor saturado de refrigerante 134a
$T_\text{sat}$ $p_\text{sat}$ $v_f$ $v_g$ $^\circ\mt F$ $\mt{psi}$ $\mt{ft^3/lb_m}$ $\mt{ft^3/lb_m}$ $15{,}37$ $30{,}0$ $0{,}012\,09$ $1{,}5492$ - Propiedades líquido-vapor saturado de amoníaco
$T_\text{sat}$ $p_\text{sat}$ $v_f$ $v_g$ $^\circ\mt C$ $\mt{bar}$ $\mt{m^3/kg}$ $\mt{m^3/kg}$ $20{,}0$ $8{,}5762$ $0{,}001\,6386$ $0{,}1492$ - $1\,\mt{ft}\equiv 30{,}48\,\mt{cm}\equiv 12\,\mt{in}$.
- $1\,\mt{lb_m}= 453{,}6\,\mt g$.
- $1\,\mt{lb_f}\equiv 1\,\mt{lb_m}\times g=4{,}45\,\mt N $.
- $1\,\mt{slug}\equiv 1\,\mt{\frac{lb_f}{ft/s^2}}= 32{,}2\,\mt{lb_m}$.
- $1\ \mt{bar}\equiv 10^5\ \mt{Pa}=2\,088{,}5\ \mt{lb/ft^2}=14{,}504\ \mt{psi}$.
-
- $n=423\ \mt{mol}$, $\bar v=1{,}18\times10^{-4}\ \mt{m^3/mol}$.
- $p=20{,}3\ \mt{MPa}$.
- $p=26{,}7\ \mt{MPa}$ , $Z=1{,}32$.
- $p=24{,}3\ \mt{MPa}$ , $Z=1{,}20$.
-
- 884 globos.
- $m=8{,}33\ \mt{kg}$.
- $\bar v=4{,}80\times 10^{-5}\ \mt{m^3/mol}$.
-
- $n_{_{\ce{O2}}}=28\ \mt{mol}$.
- $\bar v_{_{\ce{O2}}}=1{,}7\times 10^{-4}\ \mt{m^3/mol}=0{,}17\ \mt{m^3/kmol}$.
- $p_{_{0{,}0^\circ\mt{C}}}=14\ \mt{MPa}$.
- $p_{_{500^\circ\mt{C}}}=38\ \mt{MPa}$.
-
- Isobárico a $p_\text{atm}$.
- $\Delta V=1{,}0\times10^2\ \mt{ft^3}$.
- $\Delta m=7{,}4\ \mt{lb}=2{,}3\times 10^{-1}\ \mt{slug}$.
- $V=3{,}67\ \mt{cm^3}$.
-
- Diagrama $pT$
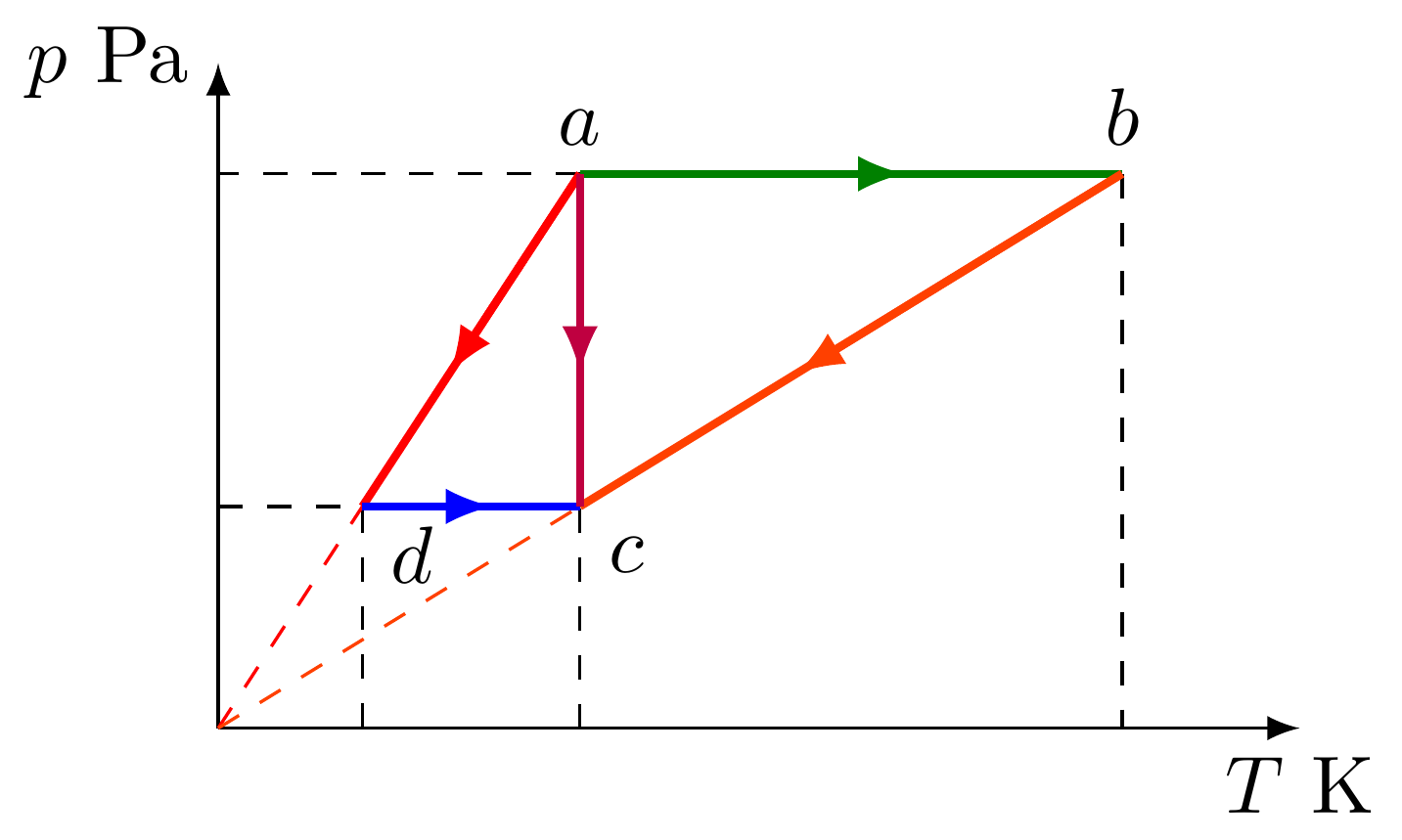
Diagrama $T\bar v$
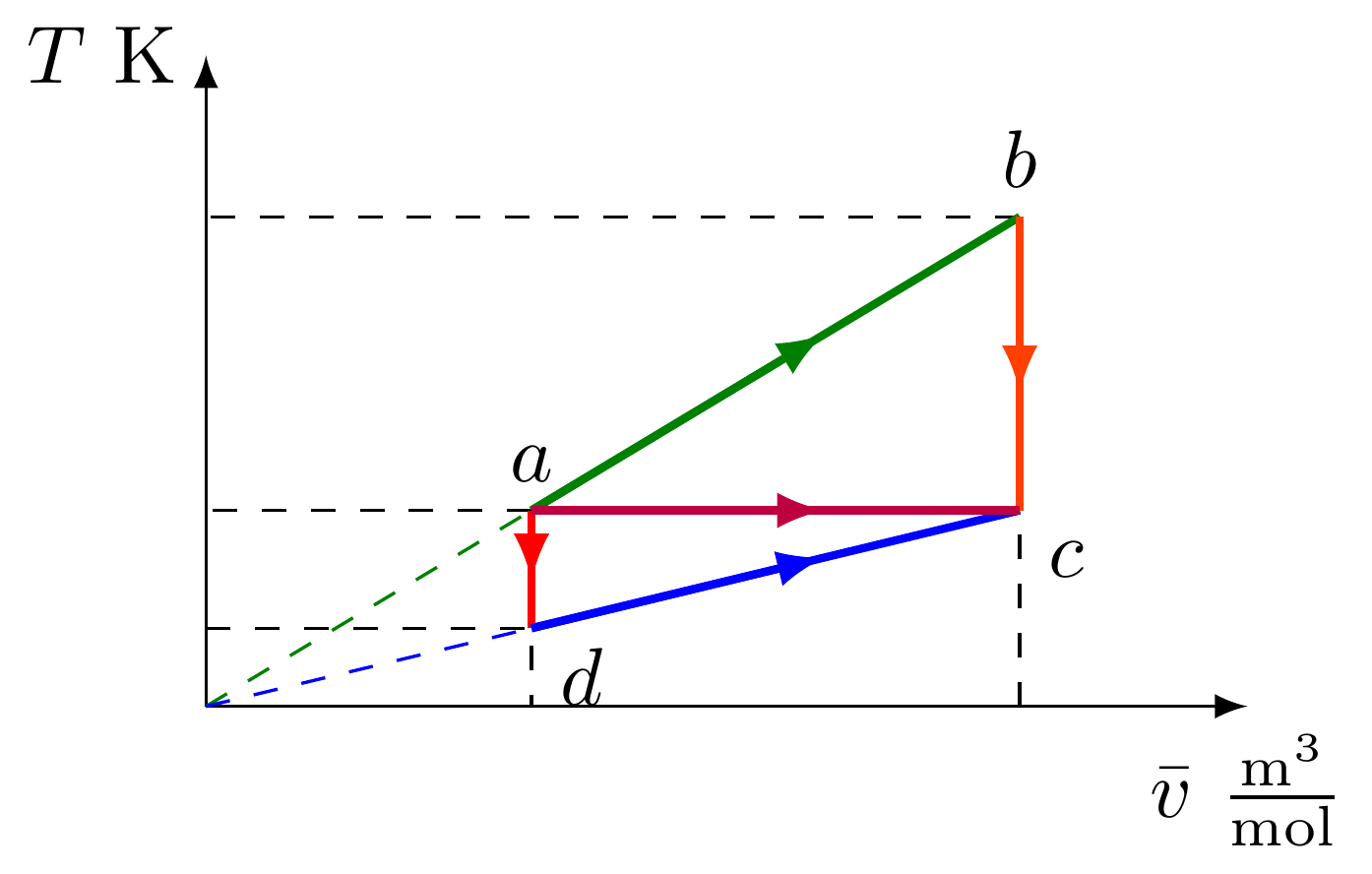
- Coordenadas termodinámicas
Estado $p$ $\bar v$ $T$ $\mt{MPa}$ $\mt{m^3/kmol}$ $\mt K$ $a$ $1{,}00$ $2{,}50$ $301$ $b$ $1{,}00$ $6{,}25$ $752$ $c$ $0{,}400$ $6{,}25$ $301$ $d$ $0{,}400$ $2{,}50$ $120$ - $V_a=10{,}0\ \mt{m^3}$.
- $m_{_{\ce{H2}}}=8{,}06\ \mt{kg}$.
- Diagrama $pT$
-
- Vapor sobrecalentado.
- Mezcla bifásica.
- Vapor sobrecalentado.
- Líquido comprimido.
- Sólido comprimido.
- Mezcla bifásica.
- Vapor sobrecalentado.
- Líquido comprimido.
- Vapor sobrecalentado.
- Sólido comprimido.
-
- $X_{\ce{H_2O}}=0{,}744$.
- $X_\text{R134a}=0{,}70$.
- $X_{\ce{N_2}}=0{,}71$.
-
- $m_{\ce{NH_3}}=128\ \mt{kg}$.
- $P=857{,}62\ \mt{kPa}$.
- $m_g=2{,}6\ \mt{lb_m}=0{,}082\ \mt{slug}$, $m_f=0{,}88\ \mt{lb_m}=0{,}027\ \mt{slug}$.
- $v=0{,}025\ \mt{\frac{m^3}{kg}}$.
Respuestas
Gases
Gases ideales
Titulación
Propiedades del agua saturada
Tabla de temperaturas SI del agua saturada
$T\ ^\circ\mt{C}$ $p_\text{sat}\ \mt{kPa}$ $v_f\ \mt{\frac{m^3}{kg}}$ $v_g\ \mt{\frac{m^3}{kg}}$ $0{,}01$ $0{,}6117$ $0{,}001\,000$ $206{,}00$ $5$ $0{,}8725$ $0{,}001\,000$ $147{,}03$ $10$ $1{,}2281$ $0{,}001\,000$ $106{,}32$ $15$ $1{,}7057$ $0{,}001\,001$ $77{,}885$ $20$ $2{,}3392$ $0{,}001\,002$ $57{,}762$ $25$ $3{,}1698$ $0{,}001\,003$ $43{,}340$ $30$ $4{,}2469$ $0{,}001\,004$ $32{,}879$ $35$ $5{,}6291$ $0{,}001\,006$ $25{,}205$ $40$ $7{,}3851$ $0{,}001\,008$ $19{,}515$ $45$ $9{,}5953$ $0{,}001\,010$ $15{,}251$ $50$ $12{,}352$ $0{,}001\,012$ $12{,}026$ $55$ $15{,}763$ $0{,}001\,015$ $9{,}5639$ $60$ $19{,}947$ $0{,}001\,017$ $7{,}6670$ $65$ $25{,}043$ $0{,}001\,020$ $6{,}1935$ $70$ $31{,}202$ $0{,}001\,023$ $5{,}0396$ $75$ $38{,}597$ $0{,}001\,026$ $4{,}1291$ $80$ $47{,}416$ $0{,}001\,029$ $3{,}4053$ $85$ $57{,}868$ $0{,}001\,032$ $2{,}8261$ $90$ $70{,}183$ $0{,}001\,036$ $2{,}3593$ $95$ $84{,}609$ $0{,}001\,040$ $1{,}9808$ $100$ $101{,}42$ $0{,}001\,043$ $1{,}6720$ $105$ $120{,}90$ $0{,}001\,047$ $1{,}4186$ $110$ $143{,}38$ $0{,}001\,052$ $1{,}2094$ $115$ $169{,}18$ $0{,}001\,056$ $1{,}0360$ $120$ $198{,}67$ $0{,}001\,060$ $0{,}891\,33$ $125$ $232{,}23$ $0{,}001\,065$ $0{,}770\,12$ $130$ $270{,}28$ $0{,}001\,070$ $0{,}668\,08$ $135$ $313{,}22$ $0{,}001\,075$ $0{,}581\,79$ $140$ $361{,}53$ $0{,}001\,080$ $0{,}508\,50$ $145$ $415{,}68$ $0{,}001\,085$ $0{,}446\,00$ $150$ $476{,}16$ $0{,}001\,091$ $0{,}392\,48$ $155$ $543{,}49$ $0{,}001\,096$ $0{,}346\,48$ $160$ $618{,}23$ $0{,}001\,102$ $0{,}306\,80$ $165$ $700{,}93$ $0{,}001\,108$ $0{,}272\,44$ $170$ $792{,}18$ $0{,}001\,114$ $0{,}242\,60$ $175$ $892{,}60$ $0{,}001\,121$ $0{,}216\,59$ $180$ $1002{,}8$ $0{,}001\,127$ $0{,}193\,84$ $185$ $1123{,}5$ $0{,}001\,134$ $0{,}173\,90$ $190$ $1255{,}2$ $0{,}001\,141$ $0{,}156\,36$ $195$ $1398{,}8$ $0{,}001\,149$ $0{,}140\,89$ $200$ $1554{,}9$ $0{,}001\,157$ $0{,}127\,21$ $205$ $1724{,}3$ $0{,}001\,164$ $0{,}115\,08$ $210$ $1907{,}7$ $0{,}001\,173$ $0{,}104\,29$ $215$ $2105{,}9$ $0{,}001\,181$ $0{,}094\,680$ $220$ $2319{,}6$ $0{,}001\,190$ $0{,}086\,094$ $225$ $2549{,}7$ $0{,}001\,199$ $0{,}078\,405$ $230$ $2797{,}1$ $0{,}001\,209$ $0{,}071\,505$ $235$ $3062{,}6$ $0{,}001\,219$ $0{,}065\,300$ $240$ $3347{,}0$ $0{,}001\,229$ $0{,}059\,707$ $245$ $3651{,}2$ $0{,}001\,240$ $0{,}054\,656$ $250$ $3976{,}2$ $0{,}001\,252$ $0{,}050\,085$ $255$ $4322{,}9$ $0{,}001\,263$ $0{,}045\,941$ $260$ $4692{,}3$ $0{,}001\,276$ $0{,}042\,175$ $265$ $5085{,}3$ $0{,}001\,289$ $0{,}038\,748$ $270$ $5503{,}0$ $0{,}001\,303$ $0{,}035\,622$ $275$ $5946{,}4$ $0{,}001\,317$ $0{,}032\,767$ $280$ $6416{,}6$ $0{,}001\,333$ $0{,}030\,153$ $285$ $6914{,}6$ $0{,}001\,349$ $0{,}027\,756$ $290$ $7441{,}8$ $0{,}001\,366$ $0{,}025\,554$ $295$ $7999{,}0$ $0{,}001\,384$ $0{,}023\,528$ $300$ $8587{,}9$ $0{,}001\,404$ $0{,}021\,659$ $305$ $9209{,}4$ $0{,}001\,425$ $0{,}019\,932$ $310$ $9865{,}0$ $0{,}001\,447$ $0{,}018\,333$ $315$ $10\,556$ $0{,}001\,472$ $0{,}016\,849$ $320$ $11\,284$ $0{,}001\,499$ $0{,}015\,470$ $325$ $12\,051$ $0{,}001\,528$ $0{,}014\,183$ $330$ $12\,858$ $0{,}001\,560$ $0{,}012\,979$ $335$ $13\,707$ $0{,}001\,597$ $0{,}011\,848$ $340$ $14\,601$ $0{,}001\,638$ $0{,}010\,783$ $345$ $15\,541$ $0{,}001\,685$ $0{,}009\,772$ $350$ $16\,529$ $0{,}001\,741$ $0{,}008\,806$ $355$ $17\,570$ $0{,}001\,808$ $0{,}007\,872$ $360$ $18\,666$ $0{,}001\,895$ $0{,}006\,950$ $365$ $19\,822$ $0{,}002\,015$ $0{,}006\,009$ $370$ $21\,044$ $0{,}002\,217$ $0{,}004\,953$ $373{,}95$ $22\,064$ $0{,}003\,106$ $0{,}003\,106$ Tabla de presiones SI del agua saturada
$p\ \mt{kPa}$ $T_\text{sat}\ ^\circ\mt{C}$ $v_f\ \mt{\frac{m^3}{kg}}$ $v_g\ \mt{\frac{m^3}{kg}}$ $1{,}0$ $6{,}97$ $0{,}001\,000$ $129{,}19$ $1{,}5$ $13{,}02$ $0{,}001\,001$ $87{,}964$ $2{,}0$ $17{,}50$ $0{,}001\,001$ $66{,}990$ $2{,}5$ $21{,}08$ $0{,}001\,002$ $54{,}242$ $3{,}0$ $24{,}08$ $0{,}001\,003$ $45{,}654$ $4{,}0$ $28{,}96$ $0{,}001\,004$ $34{,}791$ $5{,}0$ $32{,}87$ $0{,}001\,005$ $28{,}185$ $7{,}5$ $40{,}29$ $0{,}001\,008$ $19{,}233$ $10$ $45{,}81$ $0{,}001\,010$ $14{,}670$ $15$ $53{,}97$ $0{,}001\,014$ $10{,}020$ $20$ $60{,}06$ $0{,}001\,017$ $7{,}6481$ $25$ $64{,}96$ $0{,}001\,020$ $6{,}2034$ $30$ $69{,}09$ $0{,}001\,022$ $5{,}2287$ $40$ $75{,}86$ $0{,}001\,026$ $3{,}9933$ $50$ $81{,}32$ $0{,}001\,030$ $3{,}2403$ $75$ $91{,}76$ $0{,}001\,037$ $2{,}2172$ $100$ $99{,}61$ $0{,}001\,043$ $1{,}6941$ $101{,}325$ $99{,}97$ $0{,}001\,043$ $1{,}6734$ $125$ $105{,}97$ $0{,}001\,048$ $1{,}3750$ $150$ $111{,}35$ $0{,}001\,053$ $1{,}1594$ $175$ $116{,}04$ $0{,}001\,057$ $1{,}0037$ $200$ $120{,}21$ $0{,}001\,061$ $0{,}885\,78$ $225$ $123{,}97$ $0{,}001\,064$ $0{,}793\,29$ $250$ $127{,}41$ $0{,}001\,067$ $0{,}718\,73$ $275$ $130{,}58$ $0{,}001\,070$ $0{,}657\,32$ $300$ $133{,}52$ $0{,}001\,073$ $0{,}605\,82$ $325$ $136{,}27$ $0{,}001\,076$ $0{,}561\,99$ $350$ $138{,}86$ $0{,}001\,079$ $0{,}524\,22$ $375$ $141{,}30$ $0{,}001\,081$ $0{,}491\,33$ $400$ $143{,}61$ $0{,}001\,084$ $0{,}462\,42$ $450$ $147{,}90$ $0{,}001\,088$ $0{,}413\,92$ $500$ $151{,}83$ $0{,}001\,093$ $0{,}374\,83$ $550$ $155{,}46$ $0{,}001\,097$ $0{,}342\,61$ $600$ $158{,}83$ $0{,}001\,101$ $0{,}315\,60$ $650$ $161{,}98$ $0{,}001\,104$ $0{,}292\,60$ $700$ $164{,}95$ $0{,}001\,108$ $0{,}272\,78$ $750$ $167{,}75$ $0{,}001\,111$ $0{,}255\,52$ $800$ $170{,}41$ $0{,}001\,115$ $0{,}240\,35$ $850$ $172{,}94$ $0{,}001\,118$ $0{,}226\,90$ $900$ $175{,}35$ $0{,}001\,121$ $0{,}214\,89$ $950$ $177{,}66$ $0{,}001\,124$ $0{,}204\,11$ $1\,000$ $179{,}88$ $0{,}001\,127$ $0{,}194\,36$ $1\,100$ $184{,}06$ $0{,}001\,133$ $0{,}177\,45$ $1\,200$ $187{,}96$ $0{,}001\,138$ $0{,}163\,26$ $1\,300$ $191{,}60$ $0{,}001\,144$ $0{,}151\,19$ $1\,400$ $195{,}04$ $0{,}001\,149$ $0{,}140\,78$ $1\,500$ $198{,}29$ $0{,}001\,154$ $0{,}131\,71$ $1\,750$ $205{,}72$ $0{,}001\,166$ $0{,}113\,44$ $2\,000$ $212{,}38$ $0{,}001\,177$ $0{,}099\,587$ $2\,250$ $218{,}41$ $0{,}001\,187$ $0{,}088\,717$ $2\,500$ $223{,}95$ $0{,}001\,197$ $0{,}079\,952$ $3\,000$ $233{,}85$ $0{,}001\,217$ $0{,}066\,667$ $3\,500$ $242{,}56$ $0{,}001\,235$ $0{,}057\,061$ $4\,000$ $250{,}35$ $0{,}001\,252$ $0{,}049\,779$ $5\,000$ $263{,}94$ $0{,}001\,286$ $0{,}039\,448$ $6\,000$ $275{,}59$ $0{,}001\,319$ $0{,}032\,449$ $7\,000$ $285{,}83$ $0{,}001\,352$ $0{,}027\,378$ $8\,000$ $295{,}01$ $0{,}001\,384$ $0{,}023\,525$ $9\,000$ $303{,}35$ $0{,}001\,418$ $0{,}020\,489$ $10\,000$ $311{,}00$ $0{,}001\,452$ $0{,}018\,028$ $11\,000$ $318{,}08$ $0{,}001\,488$ $0{,}015\,988$ $12\,000$ $324{,}68$ $0{,}001\,526$ $0{,}014\,264$ $13\,000$ $330{,}85$ $0{,}001\,566$ $0{,}012\,781$ $14\,000$ $336{,}67$ $0{,}001\,610$ $0{,}011\,487$ $15\,000$ $342{,}16$ $0{,}001\,657$ $0{,}010\,341$ $16\,000$ $347{,}36$ $0{,}001\,710$ $0{,}009\,312$ $17\,000$ $352{,}29$ $0{,}001\,770$ $0{,}008\,374$ $18\,000$ $356{,}99$ $0{,}001\,840$ $0{,}007\,504$ $19\,000$ $361{,}47$ $0{,}001\,926$ $0{,}006\,677$ $20\,000$ $365{,}75$ $0{,}002\,038$ $0{,}005\,862$ $21\,000$ $369{,}83$ $0{,}002\,207$ $0{,}004\,994$ $22\,000$ $373{,}71$ $0{,}002\,703$ $0{,}003\,644$ $22\,064$ $373{,}95$ $0{,}003\,106$ $0{,}003\,106$ Tabla de temperaturas USCS del agua saturada
$T\ ^\circ\mt{F}$ $p_\text{sat}\ \mt{psia}$ $v_f\ \mt{\frac{ft^3}{lb_m}}$ $v_g\ \mt{\frac{ft^3}{lb_m}}$ $32{,}018$ $0{,}088\,71$ $0{,}016\,02$ $3\,299{,}9$ $35$ $0{,}099\,98$ $0{,}016\,02$ $2\,945{,}7$ $40$ $0{,}121\,73$ $0{,}016\,02$ $2\,443{,}6$ $45$ $0{,}147\,56$ $0{,}016\,02$ $2\,035{,}8$ $50$ $0{,}178\,12$ $0{,}016\,02$ $1\,703{,}1$ $55$ $0{,}214\,13$ $0{,}016\,03$ $1\,430{,}4$ $60$ $0{,}256\,38$ $0{,}016\,04$ $1\,206{,}1$ $65$ $0{,}305\,78$ $0{,}016\,04$ $1\,020{,}8$ $70$ $0{,}363\,34$ $0{,}016\,05$ $867{,}18$ $75$ $0{,}430\,16$ $0{,}016\,06$ $739{,}27$ $80$ $0{,}507\,45$ $0{,}016\,07$ $632{,}41$ $85$ $0{,}596\,59$ $0{,}016\,09$ $542{,}80$ $90$ $0{,}699\,04$ $0{,}016\,10$ $467{,}40$ $95$ $0{,}816\,43$ $0{,}016\,12$ $403{,}74$ $100$ $0{,}950\,52$ $0{,}016\,13$ $349{,}83$ $110$ $1{,}2767$ $0{,}016\,17$ $264{,}96$ $120$ $1{,}6951$ $0{,}016\,20$ $202{,}94$ $130$ $2{,}2260$ $0{,}016\,25$ $157{,}09$ $140$ $2{,}8931$ $0{,}016\,29$ $122{,}81$ $150$ $3{,}7234$ $0{,}016\,34$ $96{,}929$ $160$ $4{,}7474$ $0{,}016\,39$ $77{,}185$ $170$ $5{,}9999$ $0{,}016\,45$ $61{,}982$ $180$ $7{,}5197$ $0{,}016\,51$ $50{,}172$ $190$ $9{,}3497$ $0{,}016\,57$ $40{,}920$ $200$ $11{,}538$ $0{,}016\,63$ $33{,}613$ $210$ $14{,}136$ $0{,}016\,70$ $27{,}798$ $212$ $14{,}709$ $0{,}016\,71$ $26{,}782$ $220$ $17{,}201$ $0{,}016\,77$ $23{,}136$ $230$ $20{,}795$ $0{,}016\,84$ $19{,}374$ $240$ $24{,}985$ $0{,}016\,92$ $16{,}316$ $250$ $29{,}844$ $0{,}017\,00$ $13{,}816$ $260$ $35{,}447$ $0{,}017\,08$ $11{,}760$ $270$ $41{,}877$ $0{,}017\,17$ $10{,}059$ $280$ $49{,}222$ $0{,}017\,26$ $8{,}6439$ $290$ $57{,}573$ $0{,}017\,35$ $7{,}4607$ $300$ $67{,}028$ $0{,}017\,45$ $6{,}4663$ $310$ $77{,}691$ $0{,}017\,55$ $5{,}6266$ $320$ $89{,}667$ $0{,}017\,65$ $4{,}9144$ $330$ $103{,}07$ $0{,}017\,76$ $4{,}3076$ $340$ $118{,}02$ $0{,}017\,87$ $3{,}7885$ $350$ $134{,}63$ $0{,}017\,99$ $3{,}3425$ $360$ $153{,}03$ $0{,}018\,11$ $2{,}9580$ $370$ $173{,}36$ $0{,}018\,23$ $2{,}6252$ $380$ $195{,}74$ $0{,}018\,36$ $2{,}3361$ $390$ $220{,}33$ $0{,}018\,50$ $2{,}0842$ $400$ $247{,}26$ $0{,}018\,64$ $1{,}8639$ $410$ $276{,}69$ $0{,}018\,78$ $1{,}6706$ $420$ $308{,}76$ $0{,}018\,94$ $1{,}5006$ $430$ $343{,}64$ $0{,}019\,10$ $1{,}3505$ $440$ $381{,}49$ $0{,}019\,26$ $1{,}2178$ $450$ $422{,}47$ $0{,}019\,44$ $1{,}0999$ $460$ $466{,}75$ $0{,}019\,62$ $0{,}995\,10$ $470$ $514{,}52$ $0{,}019\,81$ $0{,}901\,58$ $480$ $565{,}96$ $0{,}020\,01$ $0{,}817\,94$ $490$ $621{,}24$ $0{,}020\,22$ $0{,}742\,96$ $500$ $680{,}56$ $0{,}020\,44$ $0{,}675\,58$ $510$ $744{,}11$ $0{,}020\,67$ $0{,}614\,89$ $520$ $812{,}11$ $0{,}020\,92$ $0{,}560\,09$ $530$ $884{,}74$ $0{,}021\,18$ $0{,}510\,51$ $540$ $962{,}24$ $0{,}021\,46$ $0{,}465\,53$ $550$ $1\,044{,}8$ $0{,}021\,76$ $0{,}424\,65$ $560$ $1\,132{,}7$ $0{,}022\,07$ $0{,}387\,40$ $570$ $1\,226{,}2$ $0{,}022\,42$ $0{,}353\,39$ $580$ $1\,325{,}5$ $0{,}022\,79$ $0{,}322\,25$ $590$ $1\,430{,}8$ $0{,}023\,19$ $0{,}293\,67$ $600$ $1\,542{,}5$ $0{,}023\,62$ $0{,}267\,37$ $610$ $1\,660{,}9$ $0{,}024\,11$ $0{,}243\,09$ $620$ $1\,786{,}2$ $0{,}024\,64$ $0{,}220\,61$ $630$ $1\,918{,}9$ $0{,}025\,24$ $0{,}199\,72$ $640$ $2\,059{,}3$ $0{,}025\,93$ $0{,}180\,19$ $650$ $2\,207{,}8$ $0{,}026\,73$ $0{,}161\,84$ $660$ $2\,364{,}9$ $0{,}027\,67$ $0{,}144\,44$ $670$ $2\,531{,}2$ $0{,}028\,84$ $0{,}127\,74$ $680$ $2\,707{,}3$ $0{,}030\,35$ $0{,}111\,34$ $690$ $2\,894{,}1$ $0{,}032\,55$ $0{,}094\,51$ $700$ $3\,093{,}0$ $0{,}036\,70$ $0{,}074\,82$ $705{,}1$ $3\,200{,}1$ $0{,}049\,75$ $0{,}049\,75$ Tabla de presiones USCS del agua saturada
$p\ \mt{psi}$ $T_\text{sat}\ ^\circ\mt{F}$ $v_f\ \mt{\frac{ft^3}{lb_m}}$ $v_g\ \mt{\frac{ft^3}{lb_m}}$ $1$ $101{,}69$ $0{,}016\,14$ $333{,}49$ $2$ $126{,}02$ $0{,}016\,23$ $173{,}71$ $3$ $141{,}41$ $0{,}016\,30$ $118{,}70$ $4$ $152{,}91$ $0{,}016\,36$ $90{,}629$ $5$ $162{,}18$ $0{,}016\,41$ $73{,}525$ $6$ $170{,}00$ $0{,}016\,45$ $61{,}982$ $8$ $182{,}81$ $0{,}016\,52$ $47{,}347$ $10$ $193{,}16$ $0{,}016\,59$ $38{,}425$ $14{,}696$ $211{,}95$ $0{,}016\,71$ $26{,}805$ $15$ $212{,}99$ $0{,}016\,72$ $26{,}297$ $20$ $227{,}92$ $0{,}016\,83$ $20{,}093$ $25$ $240{,}03$ $0{,}016\,92$ $16{,}307$ $30$ $250{,}30$ $0{,}017\,00$ $13{,}749$ $35$ $259{,}25$ $0{,}017\,08$ $11{,}901$ $40$ $267{,}22$ $0{,}017\,15$ $10{,}501$ $45$ $274{,}41$ $0{,}017\,21$ $9{,}4028$ $50$ $280{,}99$ $0{,}017\,27$ $8{,}5175$ $55$ $287{,}05$ $0{,}017\,32$ $7{,}7882$ $60$ $292{,}69$ $0{,}017\,38$ $7{,}1766$ $65$ $297{,}95$ $0{,}017\,43$ $6{,}6560$ $70$ $302{,}91$ $0{,}017\,48$ $6{,}2075$ $75$ $307{,}59$ $0{,}017\,52$ $5{,}8167$ $80$ $312{,}02$ $0{,}017\,57$ $5{,}4733$ $85$ $316{,}24$ $0{,}017\,61$ $5{,}1689$ $90$ $320{,}26$ $0{,}017\,65$ $4{,}8972$ $95$ $324{,}11$ $0{,}017\,70$ $4{,}6532$ $100$ $327{,}81$ $0{,}017\,74$ $4{,}4327$ $110$ $334{,}77$ $0{,}017\,81$ $4{,}0410$ $120$ $341{,}25$ $0{,}017\,89$ $3{,}7289$ $130$ $347{,}32$ $0{,}017\,96$ $3{,}4557$ $140$ $353{,}03$ $0{,}018\,02$ $3{,}2202$ $150$ $358{,}42$ $0{,}018\,09$ $3{,}0150$ $160$ $363{,}54$ $0{,}018\,15$ $2{,}8347$ $170$ $368{,}41$ $0{,}018\,21$ $2{,}6749$ $180$ $373{,}07$ $0{,}018\,27$ $2{,}5322$ $190$ $377{,}52$ $0{,}018\,33$ $2{,}4040$ $200$ $381{,}80$ $0{,}018\,39$ $2{,}2882$ $250$ $400{,}97$ $0{,}018\,65$ $1{,}8440$ $300$ $417{,}35$ $0{,}018\,90$ $1{,}5435$ $350$ $431{,}74$ $0{,}019\,12$ $1{,}3263$ $400$ $444{,}62$ $0{,}019\,34$ $1{,}1617$ $450$ $456{,}31$ $0{,}019\,55$ $1{,}0324$ $500$ $467{,}04$ $0{,}019\,75$ $0{,}928\,19$ $550$ $476{,}97$ $0{,}019\,95$ $0{,}842\,28$ $600$ $486{,}24$ $0{,}020\,14$ $0{,}770\,20$ $700$ $503{,}13$ $0{,}020\,51$ $0{,}655\,89$ $800$ $518{,}27$ $0{,}020\,87$ $0{,}569\,20$ $900$ $532{,}02$ $0{,}021\,24$ $0{,}501\,07$ $1\,000$ $544{,}65$ $0{,}021\,59$ $0{,}446\,04$ $1\,200$ $567{,}26$ $0{,}022\,32$ $0{,}362\,41$ $1\,400$ $587{,}14$ $0{,}023\,07$ $0{,}301\,61$ $1\,600$ $604{,}93$ $0{,}023\,86$ $0{,}255\,16$ $1\,800$ $621{,}07$ $0{,}024\,70$ $0{,}218\,31$ $2\,000$ $635{,}85$ $0{,}025\,63$ $0{,}188\,15$ $2\,500$ $668{,}17$ $0{,}028\,60$ $0{,}130\,76$ $3\,000$ $695{,}41$ $0{,}034\,33$ $0{,}084\,60$ $3\,200{,}1$ $705{,}10$ $0{,}049\,75$ $0{,}049\,75$
Comentarios
Publicar un comentario